Abstract
Background: KMT2A-rearranged (R) ALL is a high-risk disease with a frequency of 75% in infants and 10% in children and adults with ALL and is associated with chemoresistance, relapse, and poor survival. Current intensive multiagent chemotherapy regimens induce significant side effects, yet fail to cure many patients, demonstrating continued need for novel therapeutic approaches. We performed a kinome-wide CRISPR screen and identified that DYRK1A is specifically required for the survival of KMT2A-R ALL cell. DYRK1A is a member of the dual-specificity tyrosine phosphorylation-regulated kinase family and has been reported as negatively regulator of cell proliferation.
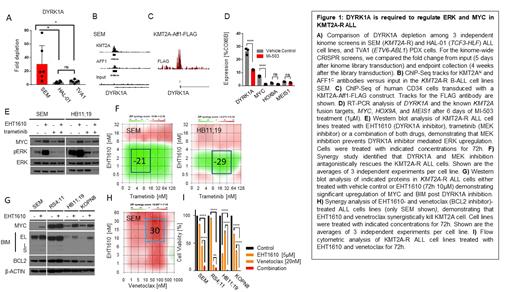
Results: We performed a kinome-wide CRISPR screen in human ALL cell lines and PDX models and identified DYRK1A as a novel target in KMT2A-R ALL. DYRK1A is a serine-threonine kinase with a proposed, but poorly defined role in cell cycle regulation. We performed a meta-analysis of multiple ChIP-Seq experiments and identified that oncogenic KMT2A fusions directly bind to the DYRK1A promoter. Our RT-PCR and Western blot analyses of KMT2A-R ALL cells treated with a menin inhibitor (MI-503) to disrupt the transcriptional activity of the KMT2A-R complex resulted in the downregulation of DYRK1A, indicating that DYRK1A is directly regulated by the KMT2A fusion complex. We further observed that pharmacologic inhibition of DYRK1A with EHT1610 induced potent leukemic cell growth inhibition in vitro and in vivo, demonstrating that DYRK1A could be a new therapeutic target in KMT2A-R ALL cells. To further elucidate the mechanism of DYRK1A function, we treated several KMT2A-R ALL cell lines in vitro with EHT1610, which surprisingly resulted in the upregulation of MYC and hyperphosphorylation of the RAS/MAPK target ERK. Given that ERK hyperactivation stops B cell proliferation during early B cell development to allow them to rearrange their B cell receptor, we hypothesized that cell cycle inhibition upon ERK hyperactivation remains as a conserved mechanism of cell cycle regulation in KMT2A-R ALL. Strikingly, combining DYRK1A inhibition with the MEK inhibitor trametinib antagonistically rescued KMT2A-R ALL cell proliferation, indicating that ERK hyperactivation is the main driver of DYRK1A inhibitor mediated cell cycle arrest. Given that DYRK1A inhibitor does not induce apoptosis and cells restart cell proliferation after EHT1610 withdrawal we concluded that a DYRK1A monotherapy may not be an ideal new treatment option. However, it has been reported that increased MYC activity induces the accumulation of BIM in Burkitt's Lymphoma. Given the increased expression of MYC following DYRK1A inhibition we performed a new Western blot analysis and validated increased expression of BIM in our KMT2A-R ALL cell lines after EHT1610 treatment. To test if targeting the interaction of BIM with BCL2 will induce an apoptotic effect when combined with EHT1610, we treated four KMT2A-R ALL cell lines with increasing concentrations of EHT1610 and the BCL2 inhibitor venetoclax. Strikingly, the combination of DYRK1A inhibition with BCL2 inhibition synergistically killed KMT2A-R ALL cells.
Conclusion: Our results validate DYRK1A as an important molecule to regulate cell proliferation via inhibition of MYC and ERK. Targeting DYRK1A results in the accumulation of BIM, which renders the cells sensitive to BCL2 inhibition via venetoclax. While further in vivo studies are needed, we predict that combining DYRK1A inhibition with venetoclax may be a novel precision medicine strategy for the treatment of KMT2A-R ALL.
Crispino: Forma Therapeutics: Research Funding; Scholar Rock: Research Funding; MPN Research Foundation: Membership on an entity's Board of Directors or advisory committees; Sierra Oncology: Consultancy. Tasian: Aleta Biotherapeutics: Consultancy; Gilead Sciences: Research Funding; Kura Oncology: Consultancy; Incyte Corporation: Research Funding. Carroll: Incyte Pharmaceuticals: Research Funding; Janssen Pharmaceutical: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal